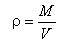
The water density ρ is defined as the ratio between the mass M of water and the volume V occupied by water:
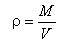 |
(2.1)
|
The water density varies with pressure, temperature and the concentration of dissolved materials. The variation of the water density with temperature is presented in Table 2.1.
Table 2.1. Variation of density of water with temperature
|
Temperature (°C) |
Water density (kg/m3) |
|
0 |
999.868 |
|
4 |
1000.000 |
|
5 |
999.992 |
|
10 |
999.727 |
|
15 |
999.126 |
|
20 |
998.230 |
For many engineering purposes the water density is taken of about 1000 kg/m3. Water density, as a function of temperature (t), may be calculated with Thiesen - Scheel - Diesselhorst relation:
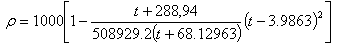 |
(2.2)
|
The specific weight of the water, γ, is defined as the ratio between the weight of the water and its volume; practically it may be obtained by multiplying the density by acceleration of gravity, g.
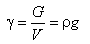 |
(2.3)
|
| where: | ||
| G | weight of the water (N). | |
| g | acceleration of gravity (ms-2) | |
For water the specific weight is of about 9810 N/m3.
The viscosity is the property of a fluid by which it develops resistance forces between two strata with relative motion. This property has been put into evidence by Newton. The space between two parallel plates is filled up with liquid; one of the plates is stationary and the other one moves in the direction x at a velocity νx. Considering that the motion of the fluid is laminar, the change of velocity upward from zero on the stationary plate to νx on the other side, respectively on the plate that moves, is linear.
The following relation gives the shear stress in x-direction, acting upon a plane having its normal in positive y-direction:
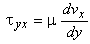 |
(2.4)
|
| where: | ||
| νx | velocity in positive x-direction | |
| τyx | shear stress | |
| dνx/dy | change of velocity with vertical direction (Figure 2.1) | |
| μ | dynamic coefficient of viscosity | |
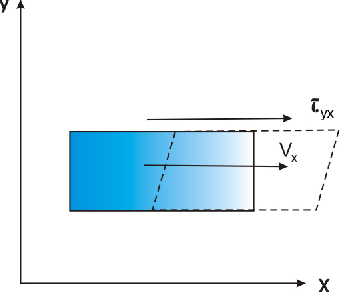
Figure 2.1. Effect of the viscosity as resistance of a fluid to shear
The dynamic coefficient of viscosity decreases with the increase of the temperature. For water, the dynamic coefficient of viscosity is of about 10-3 kg/m s.
The dynamic coefficient of viscosity is not the only possibility to quantify the viscosity of a fluid. This may be also quantified by the kinematics' coefficient of viscosity (ν), that is defined by the following relation:
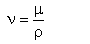 |
(2.5)
|
| where: | ||
| ν | kinematics' coefficient of viscosity | |
| ρ | density of the fluid | |
For water, the kinematics' coefficient of viscosity is of about 10-6 m2/s.
Water is only slightly compressible. At constant temperature and mass conditions, the following relation defines the isothermal compressibility of water:
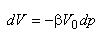 |
(2.6)
|
| where: | ||
| dV | variation of the volume of a certain amount of water (m3) | |
| Vo | initial volume of water (m3) | |
| dp | variation of the water pressures (N/m3) | |
| β | compressibility (for water the compressibility is of about 0.5 x 10-9 m2/N) | |
It should be noted that the effective compressibility of water in porous media could be much larger because of entrapped air bubbles.
The compressibility of a fluid can also be defined by using the density instead of the volume. At constant mass and temperature conditions the density of a fluid will increase by the increasing of the pressure:
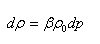 |
(2.7)
|
where p is the pressure of the fluid characterized by the initial density ρ0.
Water particles at the water table are subject to an upward attraction due to surface tension of the air-water interface and the molecular attraction of the liquid and solid phases. This phenomenon is known as capillarity.
In a tube of small diameter, the free-water surface will assume a shape with the minimum surface area. The attraction of the solid for the liquid will draw the liquid up into the tube. The upward force will be balanced by the weight of the column of the water (Figure 2.2).
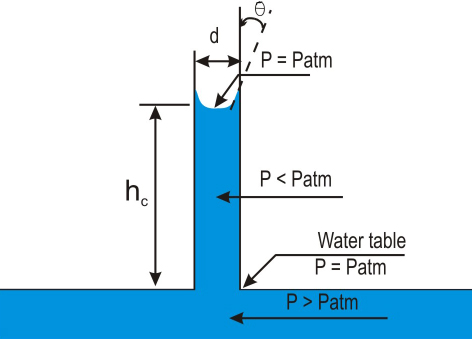
Figure 2.2. Rise of water in capillary tubes
The rise of water in a capillary tube may be calculated by the following relations:
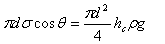 |
(2.8)
|
and
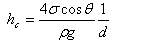 |
(2.9)
|
| where: | ||
| hc | height of the capillary rise (L) | |
| σ | surface tension coefficient of the fluid (MT-2) | |
| θ | angle of the meniscus (degree) | |
| ρ | density of the fluid (ML-3) | |
| g | acceleration of gravity (LT-2) | |
| d | diameter of the capillary tube (L) | |
There were developed special formulas that can be applied to calculate the height of capillary rise in soil, as for example Mavis and Tsui's formula (1939), that takes in consideration only the size of grain and the porosity (Delleur, 1998):
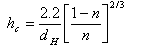 |
(2.10)
|
| where: | ||
| hc | height of the capillary rise (mm) | |
| dH | harmonic mean value of grain size diameter (mm) | |
| n | porosity | |
The origin of subsurface water can be explained by taking into consideration the two main ways of subsurface water accumulation:
In the upper levels of the Earth's crust, the subsurface water originates mainly from infiltration of precipitation; the natural recharge by infiltration is the main source of water in the underground formations. This type of water is widespread, playing an important role in the hydrologic cycle. In some cases, infiltration from surface waters into the ground may occur.
The presence of subsurface water is also a result of water vapour condensation in pores space. The intensity of condensation depends on the soil features (porosity and grain size distribution) and its geologic conditions, together with the physical properties of water. The importance of this water in the total budget is still relatively small.
The sedimentation water originates in the processes of diagenesis, during which residual solutions or removed waters are withdrawn from the hydrologic cycle and participate in the geologic cycle. These waters will enter again the hydrologic cycle as a result of geologic processes such as folding and compression breakdown of overlying strata.
The juvenile water is a result of magmatic processes, leading to the rise and
cooling of magma. This kind of waters played a considerable importance in the
early stages of the Earth's formation. In situ investigation shows that about
2 % of the water mass exists in the magmatic melt under the Earth's crust. Juvenile
waters do not have a significant importance in the hydrologic cycle.
Subsurface formations containing water may be divided vertically into a zone of aeration in which the pores contain both air and water and an underlying zone of saturation in which the pores are filled up with water. The boundary between the two zones is called water table.
The underground water may exist in the following forms (Figure 2.3):
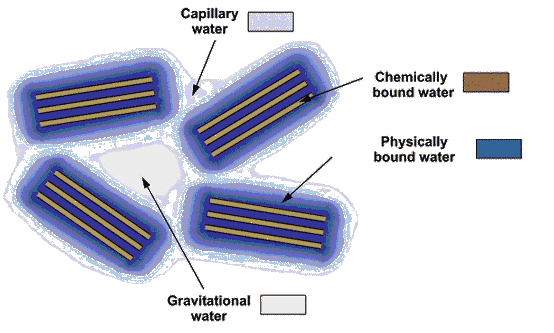
Figure 2.3. Main forms of water in soils
Chemically bound water can be classified in two categories: crystallized water and constitutional water (Galperin et al, 1993).
The crystallized water exists in the crystal lattice of minerals as a single molecule or group of molecules such as Na2 CO3 10H2O (64% of it representing H2O), Na2SO4 10H2O (55% being H2O), Ca SO4 2H2O (55% representing H2O).
The crystallized water can be separated from minerals at temperatures up to 300°C; a mineral can loose a part of its water or all water function of temperature. While heating it gradually to 170°C, gypsum partly looses water; all water is lost at a temperature of 170°C.
Constitutional water is formed by ions of OH- and H- lost by minerals containing hydroxides such as Al(OH)3 or Ca(OH)2:
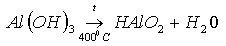 |
(2.11)
|
The generation of such water is attributed to high temperatures and pressure. Clayey minerals such as kaolinite and montmorilonite contain considerable amounts of water, which is liberated at temperatures of 460 - 550°C.
A particular type of water is plasma also named supercritical plasma (Galperin et al, 1993). This phase is generated at supercritical temperatures and pressures, occurring in the Earth's mantle. The velocity of water molecules is close to gas velocities; high mobility of the fluid and very high solving capacity characterize this water. At such parameters the differences between physical properties of liquid and vapours disappear.
Physically bound water is formed from hygroscopic or adsorbed water and from pellicular water. This water, also known as molecular water represents the molecular moisture content and forms more strata (rows) around the surface of mineral particles.
Hygrospcopic water is firmly bound on fine dispersed soils. It occurs at the surface of mineral particles, due to the adsorption of water molecules from water vapour or from liquid water. The adsorption is explained by the fact that the mineral particles have negative charges, while water molecules are dipoles with positive and negative charges. Thus electro-molecular forces of interactions between the soil and liquid phases are generated. As the dispersion of soil increases, the specific surface also increases, and as a consequence the number of the interacting water molecules grows. The electro-molecular forces of interaction at the surface of a mineral particle make up for the first row of bound water molecules.
Above this row another layer of bound water is formed; it is called intermediate layer respectively osmotic water. This water has a small mobility; it can be removed from the layer by heating the soil sample to 100 - 120°C.
Around the layer of hygroscopic water, pellicular water is found when the soil water content exceeds the maximum hygroscopic content. This layer can move away from the particle with thicker film towards particles with thinner films; the motion also occurs if there is a difference of ionic concentration in the water film.
The temperature and the pressure of the underground environment govern the equilibrium between the total amount of water and the thickness of films, respectively the amount of physically bound water. As the temperature and the pressure are higher, as the amount of physically bound water is lower, due to its transition to free water phase. Field data demonstrate that at temperatures of 100 - 150°C, respectively at depth over 1500 - 4000 m all bound water changes into free water phase (Galperin et al, 1993).
Free liquid phase water is formed by capillary water and gravitational water.
Suspended and raised capillary water is filling the canals made by the small pores in communication under the effect of water surface tension. The suspended capillary water occurs in the upper part of the aeration zone due to the infiltration of atmospheric precipitation when the soil moisture content exceeds the maximum molecular content, or in the lower part of fine soil underlying coarse varieties. The raised capillary water is situated immediately above groundwater table.
The gravitational water moves under the effect of gravity not only in saturated media, but also in unsaturated soils when the gravity force exceeds the adsorbtion and the capillarity forces. In saturated media, the gravitational water flows from areas with a higher energy to the areas characterized by a smaller energy. The volume of gravitational water depends on the granulometric composition, pore size and the degree of separation of soil aggregates.
Except the crystallization water, all the other forms of water can be extracted in the oven at a temperature of 105°C.
Gaseous phase water is encountered practically everywhere in the subsurface environment. A small quantity of water vapour is deposited in the aeration zone, between the ground surface and the water table. The vapours in this area originate from atmosphere and the evaporation of the existing water in pores and joints of soil. The vapours may move under the gradient of pressure from the areas of higher moisture content to the areas with less moisture concentration. Vapour pressure is a function of temperature, explaining thus the generation and the vapour fluxes due to the temperature gradient. Vertical fluxes are connected with seasonal warming of rocks, while the horizontal motion of water vapour is linked to the anthropogenic changes on the soil surface such as screening and shadowing. In the overall volume of gaseous water a considerable portion belongs to the steam generated at large depths, due to the continuous increase in temperature with depth or to the exit of hot waters on the ground surface (Galperin et al, 1993).
Subsurface formations containing water may be divided vertically into the aeration zone and the saturation zone (Figure 2.4).
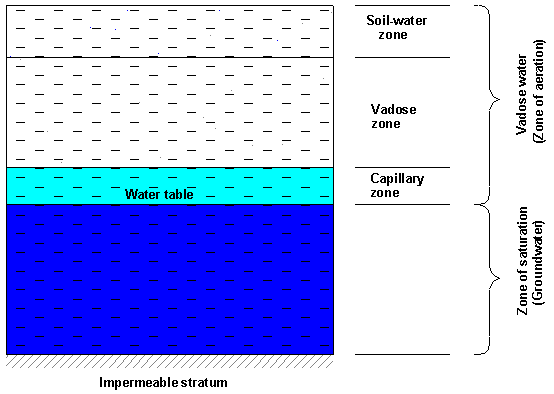
Figure 2.4. Vertical distribution zones of subsurface water
In its turn, the aeration zone is divided into three sub-zones (Bedient et al, 1994):
(a) The root zone (soil water zone), which extends from the ground surface down through the major root zone. Its extension varies with the soil type and vegetation; the moisture distribution in this zone is affected by precipitation, irrigation, air temperature and humidity and by the possible presence of a close water table (Bear, 1978). The root zone contains beside the molecular water (the bounded water) capillary water and gravitational water. Hygroscopic water remains adsorbed to the surface of soil grains; capillarity water is suspended in the canals formed by interconnected pores of small diameter, while gravitational water is drained under the influence of gravity through the big pores in communication. While the capillarity water moves in any direction (laterally, upward or downward) depending on the local conditions, the gravitational water moves downward. Temporarily, during excessive precipitation or flooding, the surface layers of the soil may be very close to saturation.
(b) The capillary zone, or fringe zone, extends from the water table up to the limit of capillary rise. The thickness of capillary fringe depends on the soil's properties and on the homogeneity of the soil, mainly on the pore size distribution. Because of the irregularities in the size of the openings, capillary water does not rise to the same height above the water table; thus, it forms an irregular fringe. The thickness of capillary fringe can range from 2,5 cm for fine gravel to more than 700 cm for silt (Table 2).
Table 2.2. Height of capillary rise in sediments
|
Sediment |
Grain diameter
(cm) |
Pore radius
(cm) |
Capillary rise
(cm) |
|
Fine silt |
0.0008 |
0.0002 |
750 |
|
Coarse silt |
0.0025 |
0.0005 |
300 |
|
Very fine sand |
0.0075 |
0.0015 |
100 |
|
Fine sand |
0.0150 |
0.003 |
50 |
|
Medium sand |
0.03 |
0.006 |
25 |
|
Coarse sand |
0.05 |
0.010 |
15 |
|
Very coarse sand |
0.20 |
0.040 |
4 |
|
Fine gravel |
0.50 |
0.100 |
1.5 |
In practical applications the upper limit of capillary fringe is considered as a smooth surface, below which the soil is assumed to be almost saturated (Bear, 1978); still the pressure is less than atmospheric. Vertical and horizontal flow of water may take place; the flow in the capillary fringe is neglected when the thickness of the saturated zone is greater than the vertical development of the capillary zone.
Within this zone there is a gradual decrease in moisture content with the height above water table, from complete saturation just above the water table to the upper limit when only the smaller connected pores are filled up with water. If the capillary fringe reaches the ground surface, it can provide directly water for evaporation. As water evaporates from the soil surface, it can be replaced by water from the zone of saturation drawn upward by capillarity.
The water can also evaporate and move through the air space in the pores as
vapors. Still, the amount of vapors water movement in the unsaturated zone is
much less important than the transport in the liquid form.
(c) The vadose zone (also called intermediate zone) extends from the
lower edge of the root zone to the upper limit of the capillary zone. The thickness
of vadose zone depends on the depth of water table below the ground surface,
so that it may vary from zero for high water table conditions to hundred of
meters in arid regions. In the vadose zone the water is permanently held in
place by hygroscopic and capillary forces; these forces cannot retain any surplus
of water coming from the root zone, which moves downward (percolation) through
the vadose zone as gravitational water.
In drainage problems, the flow in the unsaturated zones may be of primary importance (Bear, 1978).
Groundwater is a term used to denote generally the water found beneath the ground surface. In hydrogeology groundwater is the water beneath the water table (in the saturation zone), while in drainage of agricultural lands the term groundwater is sometimes used to denote the water in the partially saturated layers above the water table (Bear, 1978).
Groundwater is part of the hydrologic cycle. Very small amounts of water may enter the hydrologic cycle from other sources (magmatic waters). Precipitations infiltrate into the root zone and in certain conditions a part percolates to deeper layers; the percolation represents the main contribution to the natural recharge of the aquifers.
Infiltration capacity is defined as the maximum rate at which a soil is able to receive water in given conditions. The main factors that influence the infiltration capacity are (Barcelona et al., 1990):
The moisture content of a porous medium is a very important index giving an image about the quantity of water existing in the pore space.
The large diversity of forms of soil water makes it difficult and subjective to establish the real quantity of water. For this reason, conventionally a laboratory test is worldwide accepted. The water content laboratory test is based on the comparison of the mass of the soil before and after drying the soil sample in an oven at a temperature of 105°C. It is obvious that at this temperature a significant quantity of water remains in pores as retained water, but from the technical point of view it is considered that such conditions simulate the extreme conditions existing in nature. The incompressibility of water and solid skeleton is assumed.
The moisture content may be defined from both the mass and volumetric point of view.
The mass humidity or water content (w) is the ratio of the mass of water Mw to the mass of solids Ms in the soil dried at a temperature of 105°C, usually expressed as percentage:
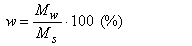 |
(2.12)
|
In many cases, a volumetric humidity or volumetric water content (θ) is required, defined as the ratio of pore water volume to the total volume of the soil sample, expressed as percentage too:
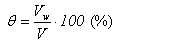 |
(2.13)
|
If only a part of the pores are full of water, the soil is unsaturated or partially saturated. Therefore a degree of saturation can be defined.
The degree of saturation Sγ or saturation ratio can be expressed in three equivalent forms:
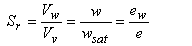 |
(2.14)
|
This relationship can also be written in terms of volumetric water content and porosity as follows:
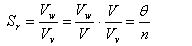 |
(2.15)
|
By using the degree of saturation, a classification of soils can be made:
Another relationship between mass humidity w and volumetric water content (θ) can be written starting from the water content finally expressed as function of the dry density of the soil:
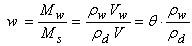 |
(2.16)
|
The air content (A) is defined as the ratio of the volume Va of pores containing air to the total volume V, being actually a kind of porosity defined only for air pores:
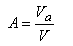 |
(2.17)
|
As it has been specified, the porous media consists on solid particles separated by pore spaces or voids, dispersed accordingly to the structure and texture of the porous medium taken into account. The pore spaces could be completely empty, partially empty or full of water. Therefore soils can be considered either two-phase or three-phase porous media. If the soil is completely dry there are only two phases: the solid soil and the pore air. A fully saturated soil is also two-phase: the solid particles and the pore water. An unsaturated (or partially saturated) soil is a three-phase medium, both pore water and pore air coexisting around the solid soil particles .
In order to establish a macro-characterization of the porous media function of the ratio of each phase, an idealization is useful to be adopted. Let's suppose that all solid particles and all pore spaces can be imaginarily separated. Thus, a phase diagram as shown in Figure 2.5 may represent the soil components schematically.
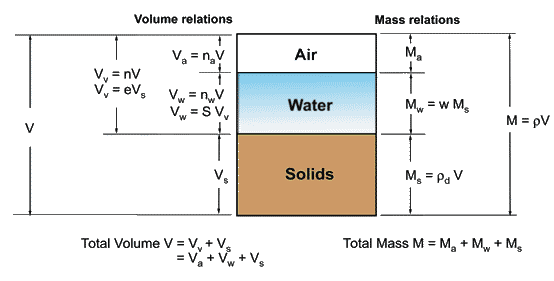
Figure 2.5. Phase diagram (after Fredlund and Rahardjo, 1993)
The bulk density (ρb) of the soil in natural conditions (the ratio of the total mass to the total volume) can be expressed as:
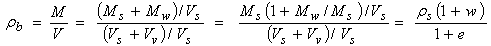 |
(2.18)
|
The bulk density can also be written using the porosity (n) and the density of solids (ρs) as following:
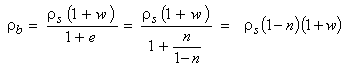 |
(2.19)
|
The dry density of the soil ρd can be obtained in a similar way:
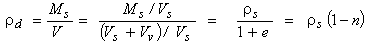 |
(2.20)
|
The same relation can be obtained if in the relation (2.19), the mass humidity w is considered null. Taking into account the relation (2.20), the relation (2.19) becomes:
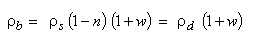 |
(2.21)
|
The density ρsat of fully saturated soils (the volume of voids is full of water, or Vw=Vv) is a linear combination of the density ρs of solid particles and the water density ρw. Indeed:
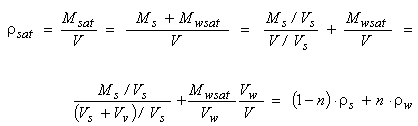 |
(2.22)
|
Finally, the air content can be expressed as:
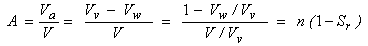 |
(2.23)
|
Bibliography
Barcelona, M., et al, 1990. Contamination of Groundwater. Prevention, Assessment, Restoration. Noyes Data Corporation, Park Ridge, New Jersey.
Bear, J., 1978. Hydraulics of groundwater. McGraw-Hill, New York.
Bedient, P., et al, 1994. Groundwater Contamination. Transport and Remediation. Prentice Hall, New Jersey.
Fredlund, D.G. and Rahardjo, H., 1993. Soil mechanics for unsaturated soils. John Willey and Sons, New York.
Galperin, M., Zayetsev, V.S. and Normatov, Y.A., 1993. Hydrogeology and Engineering Geology. A.A. Balkema, Rotterdam.