 (36 KB)
(36 KB)
Summary: Water Quality Characteristics
Introduction
Water quality is determined by physical, chemical and microbiological properties
of water. These water quality characteristics throughout the world are characterized
with wide variability.Therefore the quality of natural water sources used for
different purposes should be established in terms of the specific water-quality
parameters which most affect the possible use of water. That is why the aim
of this chapter to provides an overview of water quality characteristics - Physical
Chemical Microbiological and Biological characteristics.
Physical characteristics of water
The characteristics which determine the water quality are Physical characteristics, Chemical characteristics, Microbiological characteristics and Biological characteristics.
Physical characteristics of water (temperature, color, odor, taste, turbidity
and etc.) are determined by senses of touch,sight, smell and taste.
Chemical characteristics
The chemical characteristic of natural water are a reflection of the soils and rocks with which the water has been in contact. In addition, agricultural and urban runoff and municipal and industrial wastewater after treatment, impact the water quality. Microbial and chemical transformations also affect the chemical characteristics of water.
During the periods of contact with rocks and soils the water dissolves inorganic minerals which may dissociate to varying degrees, to cations and anions.
Major cations found in natural water include calcium (Ca2+), magnesium (Mg2+), sodium (Na2+) and potassium (K+).Calcium (Ca2+), is the most prevalent cation in water and second inorganic ion to bicarbonate in most surface water.
Other constituents in natural water in concentration of 1 mg/L or higher include aluminum, boron, iron, manganese, phosphorus and etc.
Major anions include chloride, sulfate, carbonate, bicarbonate, fluoride and nitrate. Bicarbonate (HCO3-) is the principal anion found in natural water. This ions are very important in the carbonate system, which provides a buffer capacity to natural water and is responsible in a great measure for the alkalinity of water.
Other anions found in water include fluorides (F-), carbonates (CO32-) and phosphates (PO43-).
The carbonate - bicarbonate system is presumably the most important chemical system in natural waters. The carbonate system provides the buffering capacity essential for maintaining the pH of natural water systems in the range required by bacteria and other aquatic species. The carbonate system includes the following species: CO2, H2CO3, HCO3-, CO32-, OH- and H+.The total content (mol/dm3) of its components is folowing:
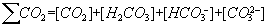
The hydrogen ion (H+) concentrations in water controls the pH of the solution. The pH of water is defined as the negative logarithm of the [H+], where [H+] is the hydrogen ion concentration expressed in moles per liter (mol/L). (1).
The hydrogen ion (H+) concentrations in water controls the pH of the solution. The pH of water is defined as the negative logarithm of the [H+], where [H+ ] is the hydrogen ion concentration expressed in moles per liter (mol/L). (1).
(1) 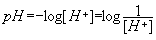 ; pOH =
- log[OH-] = log
; pOH =
- log[OH-] = log 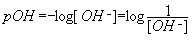
Changes in pH can have drastic effects on the species present in the carbonate system.
Alkalinity is defined as the capacity of natural water to neutralize acid added to it. Total alkalinity is the amount of acid required to reach a specific pH (pH = 4,3 to 4,8). Total alkalinity can be approximated by alkalinity as the following expression:
Total alkalinity = [HCO3-] +2[CO32-] + [OH-]- [H+].
Total alkalinity includes Hydroxide alkalinity [OH-]; Bicarbonate alkalinity [HCO3-] and Carbonate alkalinity [CO32-]. If the pH and total alkalinity are measured, the concentration of the various components of alkalinity can be calculated.
Acidity is the "quantitative capacity of aqueous media to react with hydroxyl ions". Titration with a strong base (NaOH) to defined end points (pH = 4,3 and pH=8,3).
Acidity indicates the corrosiveness of acidic water on steel, concrete and other materials.
Some of the inorganic indicators include hardness, total dissolved solids, conductivity, and adsorption ratio.
Organic chemicals are made up of carbon (C), hydrogen (H), as well as nitrogen (N) and oxygen (O). Organic compounds are derived from living organism as well as industrial sources. A wide variety of assortment of organic compounds are produced in the chemical and petrochemical industries. Organic compounds also may contain sulfur (S), phosphorus (P), fluorine (F), chlorine (CL), bromine (Br), and iodine (I).
Organic compounds in water also affect of the water quality. Organic chemicals cause disagreeable tastes and odors in drinking water. Vinyl chloride, benzene and other organic contaminants are known carcinogenic agents, while chloroform is a cancer-suspect agent. Organics in water can be expressed in terms total organic carbon (TOC).
Organic Indicators of Water Quality is Total Oxygen Demand - Chemical Oxygen Demand (COD); Biochemical Oxygen Demand and Nitrogenous Biochemical Oxygen Demand
Biological oxygen demand (BOD), the most widely used parameter is a measure of the amount of oxygen used by indigenous microbial population in water in response to the introduction of degradable organic material. The 5 - day BOD (BOD5) is most widely used. The chemical oxygen demand (COD) test of natural water yields the oxygen equivalent of the organic matter that can be oxidized by strong chemical oxidizing agent in an acidic medium.
The principal transfer of gas in natural water is the transfer of oxygen from the atmosphere to the water. However, gas transfer also is used to strip hydrogen sulfide (H2S), ammonia (NH3) and volatile organic compounds (VOC) from water. The equilibrium of each phase, concentration of gases or volatile organic compounds dissolved in water depends on the temperature, the type of gas or volatile compounds and the partial pressure of the gas or volatile compounds adjacent to the water. The relationship between the partial pressure of the gas in the atmosphere above the water and the concentration of the gas or volatile compound in the water is described by Henry, s law: Typical dissolved oxygen concentrations observed in streams and rivers throughout the world are 3 to 9 mg/l. The observed range of dissolved oxygen concentrations is 0 mg/L (anoxic conditions) and 19 mg/L (supersaturated conditions).
Dissolved oxygen is important in natural water because it is required by many microorganisms and fish in aquatic system. Dissolved oxygen also establishes an oxic environment in which oxidized forms of many constituents in water are predominant.
Under anoxic conditions in the water, reduced forms of chemical species are
formed and frequently lead to the release of undesirable odors until oxic conditions
develop.
Microbiological Characteristics
The principal groups of microorganisms in natural water include protists, plants and animals. Many bacteria, viruses and protozoa are causative organisms for some of the more virulent diseases transmitted to humans directly through water and indirectly through contaminated food. Typical diseases associated with water are listed Table…
Assay and confirmation of the presence of the causative agent of waterborne
diseases are lengthy and time consuming. In lieu of specific analyses, coliform
organisms have been used to determine the biological characteristics of natural
waters. The coliform group of bacteria are aerobic and/or facultative gram-negative,
nonspore-forming, rod-shaped bacteria that ferment lactose to gas. Escherichia
coli is commonly used as an indicator organism. This organism is present in
the intestine of warm- blooded animals, including humans.
Biological characteristics
In a typical aquatic ecosystem plant and animal materials are composed of carbon, hydrogen, oxygen, nitrogen, phosphorus and sulfur. These elements are building block for carbohydrates, lipids, proteins, phospholipids and nucleic acid.
Organic nitrogen ammonia (NH3), nitrite (NO2-), nitrate (NO3-) and nitrogen gas are important gas (N2) are important nitrogen - containing compounds in aquatic systems.
Phosphoric acid (H3PO4) which is not very volatile, can loose up to 3 H+: H3PO4 = H+ + H2PO4- ; H2PO4- = H+ + HPO42- and HPO42- = H+ + PO43-.
Sulfate occur in natural water as organic sulfur, hydrogen sulfide (H2S), elemental sulfur (S) and sulfate (SO42-). The hydrogen sulfide (H2S) is toxic to many organisms, and also is the source of odor in water.