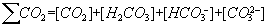
Water quality is determined by physical, chemical and microbiological properties of water. These water quality characteristics throughout the world are characterized with wide variability. Therefore the quality of natural water sources used for different purposes should be established in terms of the specific water-quality parameters that most affect the possible use of water. That is why the aim of this chapter is to provide an overview of water quality characteristics - Physical, Chemical, Microbiological, and Biological characteristics.
Physical characteristics of water (temperature, colour, taste, odour and etc.) are determined by senses of touch, sight, smell and taste. For example temperature by touch, colour, floating debris, turbidity and suspended solids by sight, and taste and odour by smell.
The temperature of water affects some of the important physical properties and characteristics of water: thermal capacity, density, specific weight, viscosity, surface tension, specific conductivity, salinity and solubility of dissolved gases and etc. Chemical and biological reaction rates increase with increasing temperature. Reaction rates usually assumed to double for an increase in temperature of 10 °C. The temperature of water in streams and rivers throughout the world varies from 0 to 35 °C.
Colour in water is primarily a concern of water quality for aesthetic reason. Coloured water give the appearance of being unfit to drink, even though the water may be perfectly safe for public use. On the other hand, colour can indicate the presence of organic substances, such as algae or humic compounds. More recently, colour has been used as a quantitative assessment of the presence of potentially hazardous or toxic organic materials in water.
Taste and odour are human perceptions of water quality. Human perception of taste includes sour (hydrochloric acid), salty (sodium chloride), sweet (sucrose) and bitter (caffeine). Relatively simple compounds produce sour and salty tastes. However sweet and bitter tastes are produced by more complex organic compounds. Human detect many more tips of odour than tastes. Organic materials discharged directly to water, such as falling leaves, runoff, etc., are sources of tastes and odour-producing compounds released during biodegradation.
Turbidity is a measure of the light-transmitting properties of water and is comprised of suspended and colloidal material. It is important for health and aesthetic reasons.
The total solids content of water is defined as the residue remaining after evaporation of the water and drying the residue to a constant weight at 103 °C to 105 °C. The organic fraction (or volatile solids content) is considered to be related to the loss of weight of the residue remaining after evaporation of the water and after ignition of the residue at a temperature of 500 °C. The volatile solids will oxidize at this temperature and will be driven off as gas. The inorganic (or fixed solids) remind as inert ash. Solids are classified as settleable solids, suspended solids and filterable solids. Settleable solids (silt and heavy organic solids) are the one that settle under the influence of gravity. Suspended solids and filterable solids are classified based on particle size and the retention of suspended solids on standard glass-fibre filters.
The chemical characteristics of natural water are a reflection of the soils and rocks with which the water has been in contact. In addition, agricultural and urban runoff and municipal and industrial treated wastewater impact the water quality. Microbial and chemical transformations also affect the chemical characteristics of water
Runoff causes erosion and weathering of geological formation, rocks and soils as the runoff travels to the surface-water bodies. During this period of contact with rocks and soils the water dissolves inorganic minerals, which enter the natural waters. Inorganic compounds may dissociate to varying degrees, to cations and anions.
Major cations found in natural water include calcium (Ca2+), magnesium (Mg2+), sodium (Na+) and potassium (K+). Calcium (Ca2+), is the most prevalent cation in water and second inorganic ion to bicarbonate in most surface water.
The principal concern about calcium is related to the fact that calcium is the primary constituent of water hardness. Calcium precipitates as CaCO3 in iron and steel pipes. A thin layer of CaCO3 can help inhibit corrosion of the metal. However, excessive accumulation of CaCO3 in boilers, hot water heaters, heat exchangers, and associated piping affects heat transfer and could lead to plugging of the piping. Calcium concentration of up to 300 mg/L or higher have been reported. However, calcium concentrations of 40 to 120 mg/L are more common.
Magnesium is not abundant in rocks as calcium. Therefore, although magnesium salts are more soluble than calcium, less magnesium is found in surface water. Sodium and potassium are commonly found as free ions. The concentration of these cations in natural water usually are low.
Other constituents in natural water in concentration of 1 mg/L or higher include aluminium, boron, iron, manganese, phosphorus and etc.
Major anions include chloride, sulfate, carbonate, bicarbonate, fluoride and nitrate. Bicarbonate (HCO3-) is the principal anion found in natural water. These ions are very important in the carbonate system, which provides a buffer capacity to natural water and is responsible in a great measure for the alkalinity of water.
One source of bicarbonate ions (HCO3-) in natural water is the dissociation of carbonic acid (H2CO3) that is formed when carbon dioxide (CO2) from the atmosphere, or from animal (e.g. fish) and bacterial respiration, dissolves in water.
In addition to bicarbonates (HCO3-) anions such as chlorides (Cl-), sulfates (SO42-), and nitrates (NO3-) are commonly found in natural water. These anions are released during the dissolution and dissociation of common salt deposits in geologic formations.
The concentration of the chlorides anions (Cl-) determines the water quality because the quality of water get worse after increasing in the concentration of this anions which limit possibilities of using of natural water for different purposes (household, agriculture, industry and etc.). Principal source of the chlorides anions (Cl-) in natural water are magmatic rock formations that include chlorine-content minerals. The second source of this anions is Ward Ocean from where a considerably amount of chlorides anions (Cl-) enter in the atmosphere. From atmosphere chlorides anions (Cl-) enter in the natural water in result of interaction between precipitation and soil.
The sulfates anions (SO42-) are frequently found in natural water as the result of the chemical dissolution, dissolve sulfur-content minerals and oxidize sulfates and sulfur:
CaCO3 + H2SO4 = CaSO4 + H2O+CO2; 2FeS2 + 7O2 + 2H2O = 2FeSO4 + 2H2SO4; 2S + 3O2+ 2 H2O = 2 H2SO4. |
(2.1) |
The sulfates anions (SO42-) enter in natural water as the result of the oxidation of the substances from plant and animal origin. The increase concentration of the sulfates anions (SO42-), at one hand brings about change for the worse of some physical characteristics of water (taste, smell and etc.) and on the other hand has destructive influence upon human consumption. The concentration of the sulfates anions (SO42-) fluctuates in a wide range in surface water - from 5 mg/l to 60 mg/l.
Nitrate anions (NO3) are found in natural water as the result of the bacteriological oxidation of nitrogenous materials in soil. That is why the concentration of these anions rapidly increases in summer when the process of the nitrification takes place very intensively. Another important source for dressing of the surface water with Nitrate anions (NO3) are precipitations, which absorb nitric oxides and convert them into nitric acid. A great deal of nitrate anions (NO3) enter in surface water together with domestic water and water from industry, agriculture and etc. Nitrate anions (NO3) are one of the indicators for the degree of the pollution with organic nitrate-content substances.
Other anions found in water include fluorides (F-), carbonates (CO32-) and phosphates (PO43-). Typical concentrations of major ions in the classic "word average" river are presented in Table 2.1.
Table 2.1 Typical concentrations of Major Ions in the Classic
"Word Average" River (Livingstone, 1963)
| Constituent |
Concentration mg/L |
Cations meg/L |
Anions meg/L |
| Cations |
|||
| Ca2+ |
15 |
0.750 |
- |
| Mg2+ |
4.1 |
0.342 |
- |
| Na+ |
6.3 |
0.274 |
- |
| K+ |
2.3 |
0.059 |
- |
| Anions |
|||
| HCO3- |
58.4 |
- |
0.958 |
| SO42- |
11.2 |
- |
0.233 |
| Cl- |
7.8 |
- |
0.220 |
| NO3- |
1 |
- |
0.017 |
| Sum |
106.1 |
1.425 |
1.428 |
The carbonate - bicarbonate system is presumably the most important chemical system in natural waters. The carbonate system provides the buffering capacity essential for maintaining the pH of natural water systems in the range required by bacteria and other aquatic species.
The carbonate system includes the following species: CO2, H2CO3, HCO3- , CO32-, OH- and H+. The total content (mol/dm3) of its components is as follows:
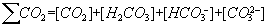 |
(2.2) |
Frequently Ca2+ and CaCO3 are included in the carbonate system, since Ca2+ is second in abundance to HCO3- in natural waters. The solution of carbon dioxide (CO2) into natural water causes the formation of carbonic acid (H2CO3) (1). The H2CO3 dissociates to bicarbonate (HCO) and hydrogen (H+) ions (2). In its turn HCO3- can dissociate and produce carbonate (CO) and hydrogen (H+) ions (3).
(1) CO2 + H2O (2) H2CO3 (3) HCO3 |
(2.3) |
The hydrogen ion (H+) concentrations [H+] = 10-pH in water controls the pH of the solution. The pH of water is defined as the negative logarithm of the [H+], where [H+] is the hydrogen ion concentration expressed in moles per litre (mol/L).
|
(2.4) |
The pH is the negative power to which 10 must be raised to equal the hydrogen ion concentration or [H+] = 10-pH. In a neutral solution [H+] is 10-7 or pH = 7. At greater hydrogen ion concentrations the pH is lower and for greater hydroxide ion concentrations the pH increases. The pH range is from 0 (extremely acidic) to 14 (extremely basic).
Therefore, the pH of the water controls which species is predominant. Water molecules HOH (commonly written as H2O) dissociate or ionize to H+ and OH- ions.
2H2O or written in a simple form: H2O |
(2.5) |
The product of [H+] and [OH-], in mol/L, is constant:
[H+] [OH-] = K = 1 x 10-14 |
(2.6) |
where K is the ion-product constant of water.
If the hydrogen ion concentration is 10-4 mol/L the hydroxide ions (OH-) concentration must be 10-14/10-4 = 10-10 mol/L. Since the 10-10 is smaller than 10-4, the solution is acidic. A large amount of hydrogen ions (H+) in water makes the water acid and lack of hydrogen ions makes the water basic. A basic solution has predominance of hydroxide ions (OH-). The dissociation reactions of carbonic acid (H2CO3) and of the bicarbonate ion (HCO) can be written as the following equations:
H2CO3 H2CO3 |
(2.7) |
These equations can be used to define the relative distribution of carbonate species as a function of pH. Changes in pH can have drastic effects on the species present in the carbonate system. Therefore the fraction of carbonic acid [H2CO3] may be expressed as α0 and written as the fraction:
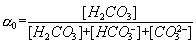 |
(2.8) |
Similarly the fraction of bicarbonate ion [HCO3-] and carbonate ions [CO32-] may be expressed as α1 and α2, respectively, and written as the following fractions:
|
(2.9) |
Alkalinity is defined as the capacity of natural water to neutralize acid added to it. Total alkalinity is the amount of acid required to reach a specific pH (pH = 4,3 to 4,8). Total alkalinity can be approximated by alkalinity as the following expression:
Total alkalinity = [HCO3-] + 2[CO32-] + [OH-] - [H+] |
(2.10) |
Total alkalinity includes Hydroxide alkalinity [OH-], Bicarbonate alkalinity [HCO3-] and Carbonate alkalinity [CO32-]. If the pH and total alkalinity are measured, the concentration of the various components of alkalinity can be calculated using the values of α1 and α2 determined for the pH of the water. These values can be used to calculate the:
Hydroxide alkalinity = Bicarbonate alkalinity = α1Ct Carbonate alkalinity = 2 α2Ct |
(2.11) |
where Ct is total carbonate and Ct = [ H2CO3] + [HCO3-] + [CO32-].
The amount of strong acid (in eq/L) required to change colour of the water from pink to clear (colourless) when a small amount of phenolphthalein reagent is put into the water sample is phenolphthalein alkalinity. This colour change occurs at approximately pH = 8.3. Continuing the titration to pH = 4.3, which is the H2CO3 endpoint, yields the total alkalinity. The values of each three forms of alkalinity can be determined using the relative values of the phenolphthalein alkalinity and the total alkalinity, expressed as either eq/L or mg/L CaCO3.
Acidity is the "quantitative capacity of aqueous media to react with hydroxyl ions". Titration with a strong base (NaOH) to define end points (pH = 4,3 and pH = 8,3). Acidity indicates the corrosiveness of acidic water on steel, concrete and other materials.
Some of the inorganic parameters include hardness, total dissolved solids, conductivity, and adsorption ratio.
Hardness is correlated with TDS (Total dissolved solids). It represents total concentration of Ca2+ and Mg2+ ions, and is reported in equivalent CaCO3. Other ions (Fe2+) may also contribute. Hardness expressed as mg/L CaCO3 is used to classify waters from "soft" to "very hard". This classification is summarized in Table 2.2.
Table 2.2 Relationship of Hardness Concentration and Classification of Natural water (F. Joseph; Jr. Malina)
|
Hardness as mg/L CaCO3
|
Classification
|
|
0 - 60
|
Soft
|
|
61 - 120
|
Moderately hard
|
|
121 - 180
|
Hard
|
|
>180
|
Very hard
|
Hardness observed for streams and rivers throughout the world ranges between 1 to 1000 mg/L. Typical concentrations are 47 mg/L to 74 mg/l CaCO3.
Hardness is an indicator to industry of potential precipitation of calcium carbonates in cooling towers and boilers, interference with soaps and dyes in cleaning and textile industries and with emulsifiers in photographic development. Hard water is less corrosive than soft. Treatment usually left to consumer (domestic, industrial, etc) depending on needs.
Total dissolved solids (TDS) is a measure of salt dissolved in a water sample after removal of suspended solids. TDS is residue remaining after evaporation of the water. The TDS load carried in streams throughout the world has been estimated by Livingston (1963) to 120 mg/L .
The concentration of total dissolved solids (TDS) is related to electrical conductivity (EC; mhos/cm) or specific conductance. The conductivity measures the capacity of water to transmit electrical current. The conductivity is a relative term and the relationship between the TDS concentration and conductivity is unique to a given water sample and in a specific TDS concentration range. The conductivity increases as the concentration of TDS increases.
TDS and conductivity affect the water sample and the solubility of slightly soluble compounds and gases in water (e.g. CaCO3, and O2). In general, the corrosiveness of the water increases as TDS and EC increase, assuming other variables are kept constant.
The Sodium Adsorption Ratio (SAR) is used to evaluate the hazard in irrigation waters caused by sodium (Na+). The SAR relates the concentration of sodium ions to the concentration of magnesium (Mg2+) and calcium (Ca2+) ions. The SAR is defined as:
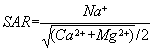 in meg/L
in meg/L |
(2.12) |
The proper ratio of sodium ions to calcium and magnesium ions in irrigation water results in irrigated soil that is granular in texture, easily worked, and permeable. With increasing proportions of sodium as the SAR increases, soil tends to become less permeable and more difficult to work.
Radionuclides in water are classified according to the type of energy released - alpha radiation (positively charged helium nuclei); beta radiation (electrons) or gamma radiation (electromagnetic energy).
Natural radiation is found in elements in the Earth's crust (potassium-40 (40K)). Another source of natural radiation results from cosmic ray bombardment in the atmosphere (tritium (3H) and carbon-14 (14C)). Other high-atomic-weight, naturally occurring isotopes found in natural water include uranium-238, thorium-232, uranium-235 and breakdown products as radium-226 and radium-228.
The units of radiation measurements are curies (CI) or rems (CI = 3,7 X 1010 nuclear transformations per second; picocurie (pCI) = 10-12 CI). A rem is the radiation dose producing the same biological effect (rem = absorbed dose X quality factors).
Each type of radiation has different health effects. For example, alpha particles travel at velocities up 107 m/sec. When ingested the relatively massive alpha particles can be very damaging to body tissue. Beta particles travel at about the speed of light, penetrate to greater depth because of their smaller mass and create less damage. Gamma radiation penetrates deeply, but has limited effects at low levels. The body dose that accurses from drinking water compared to natural background radiation is low, however, EPA policy assumes that potential harm exists from any level of radiation.
Organic chemicals are made up of carbon (C), hydrogen (H), as well as nitrogen (N) and oxygen (O). Organic compounds are derived from living organism as well as industrial sources. A wide variety of assortments of organic compounds are produced in the chemical and petrochemical industries. Organic compounds also may contain sulfur (S), phosphorus (P), fluorine (F), chlorine (Cl), bromine (Br), and iodine (I).
Organic compounds in water also affect the water quality. Organic chemicals cause disagreeable tastes and odours in drinking water. Vinyl chloride, benzene and other organic contaminants are known carcinogenic agents, while chloroform is a cancer-suspect agent.
Organic materials are found in natural water as a result of a wide range of processes, together with precipitation and surface water, as the result of the interaction between soils and precipitation and etc. Organic materials in soils originate from plant and animal degradation products. These degradation products condense and polymerise into fulvic and humic acids to kerogen and finally coal. Chemical and microbial processes cause the transformation by first attacking functional groups and aliphatic side chains. Condensation and polymerisation of various reactive groups result in larger, more aromatic molecules that decrease in solubility until kerogen or humin is produced. The end products are not soluble in acid or alkali and are resistant to biodegradation and chemical reaction.
Synthetic organic compounds include a broad variety of aliphatic and aromatic compounds. Many manufactured organic compounds may be found at very low concentrations in natural water. Isolation, identification and evaluation of health effects of these synthetic organics at low concentrations are lacking.
Organics in water can be expressed in terms total organic carbon (TOC). The TOC is the difference between total carbon (TC) and inorganic carbon (IC). Typical concentrations of organic matter observed in natural water in streams and rivers throughout the world are presented in Table 2.3.
Table 2.3 Water quality of streams and rivers and Organic materials
(Mc. Culcheon (1993); Livingstone (1963); Hem (1971))
|
Water quality parameter
|
Typical value, mg/L
|
Observed ranges, mg/L
|
|
Inorganic carbon (IC)
|
50
|
5-250
|
|
Total organic carbon (TOC)
|
1-10
|
0.01 - 40
|
|
Dissolved organic carbon (DOC)
|
1-6
|
0.3-32
|
|
Volatile organic carbon (VOC)
|
0.05
|
|
|
Total organic matter
|
2-20
|
0.02 - 80
|
For quantity assessment of concentrations of organic materials, indicator Total Oxygen Demand are used. The Total Oxygen Demand includes Chemical Oxygen Demand (COD); Biochemical Oxygen Demand and Nitrogenous Biochemical Oxygen Demand and can be shown as :
CaHbOcNdSe
+ xO2 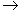 aCO2 + ½ bH2O + dNO3-
+ eSO4-
aCO2 + ½ bH2O + dNO3-
+ eSO4- |
(2.13) |
Biological oxygen demand (BOD), the most widely used parameter, is a measure of the amount of oxygen used by indigenous microbial population in water in response to the introduction of degradable organic material. This parameter depends on water characteristics: dilution, essential nutrients (N, P, K, Fe, etc), and bacteria seed. The 5-day BOD (BOD5) is most widely used. The BOD5 of natural water is related to the dissolved oxygen concentration, which is measured at zero time and after 5 days of incubation at 20 °C. The difference is the dissolved oxygen used by the microorganisms in the biochemical oxidation of organic matter. The BOD5 can be calculated as BOD5 = D0 - D1, in which the BOD5 is in mg/L and D0 and D1 are the dissolved oxygen concentration in mg/L at time 0 and 5 days, respectively.
Typical concentration of BOD5 for streams and rivers throughout the world are < 2 to 15 mg/ L and the observed range is < 2 to 65 mg/L.
The chemical oxygen demand (COD) test of natural water yields the oxygen equivalent of the organic matter that can be oxidized by strong chemical oxidizing agent in an acidic medium. Potassium permanganate is the oxidizing chemical. Silver sulfate is added as a catalyst and to minimize the interference of chloride on the COD test. Mercuric sulfate is also added to inhibit interferences of metals on the oxidation of organic compounds. The reaction of the dichromate with organic matter is presented here in general way:
Organic matter (CaHbOc)
+ Cr2O72- + H- 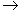 2Cr3+ + CO2 + H2O
2Cr3+ + CO2 + H2O |
(2.14) |
The COD observed in natural streams and rivers is < 2 mg/L to 100 mg/L.
The principal transfer of gas in natural water is the transfer of oxygen from the atmosphere to the water. However, gas transfer is also used to strip hydrogen sulfide (H2S), ammonia (NH3) and volatile organic compounds (VOC) from water. In both processes material is transferred from one bulk phase to another across a gas-liquid interface. For example , oxygen is transferred from the bulk gaseous phase (atmosphere) across the gas-liquid interface into bulk liquid phase (water). In the case of striping volatile organic compounds (VOC) from liquid, the VOC is transferred from the bulk liquid phase (water) across the liquid-gas interface into the bulk gaseous phase (atmosphere).
The equilibrium of each phase, concentration of gases or volatile organic compounds dissolved in water, depends on the temperature, the type of gas or volatile compounds, and the partial pressure of the gas or volatile compounds adjacent to the water. The relationship between the partial pressure of the gas in the atmosphere above the water and the concentration of the gas or volatile compound in the water is described by Henry's law:
| Xg = H Pg | (2.15) |
| where: | ||
| Pg | partial pressure of gas, atm.; | |
| H | Henry's law constant; | |
| Xg | equilibrium mole fraction of dissolved gas, mol/dm3. | |
The Henry's law constant is a function of type of gas or volatile organic compounds, temperature of the bulk liquid and constituent of the liquid (water). Values of Henry's constant for various gases that are slightly soluble in water at 20 °C are shown in Table 2.4. Thermodynamic parameters that are required to adjust Henry's law constants for different temperatures are also include in Table 2.4.
Table 2.4 Henry's Law Constants for Selected Gases that are
Slightly Soluble in Water (James M. Montgomery, 1985)
|
Gas
|
Henry's constant at 20 °C, atm
|
Temperature dependence
|
|
|
ΔH, kcal/kmol ·
10-3
|
K
|
||
|
Oxygen (O2)
|
4.3
|
1.45
|
7.11
|
|
Nitrogen (N2)
|
8.6
|
1.12
|
6.85
|
|
Methane (CH4)
|
3.8
|
1.54
|
7.22
|
|
Ozone (O3)
|
5.0
|
2.52
|
8.05
|
|
Carbon dioxide (CO2)
|
1.51
|
2.07
|
6.73
|
|
Hydrogen sulfide (H2S)
|
5.15
|
1.85
|
5.88
|
|
Chlorine (Cl2)
|
5.85
|
1.74
|
5.75
|
|
Chlorine dioxide (CLO2)
|
54
|
2.93
|
6.76
|
|
Sulfur dioxide (SO2)
|
38
|
2.40
|
5.68
|
|
Ammonia (NH3)
|
0.76
|
3.75
|
6.31
|
Typical dissolved oxygen concentrations observed in streams and rivers throughout the world are 3 to 9 mg/l. The observed range of dissolved oxygen concentrations is 0 mg/L (anoxic conditions) to 19 mg/L (supersaturated conditions).
Dissolved oxygen is important in natural water because many microorganisms and fish require it in aquatic system. Dissolved oxygen also establishes an aerobic environment in which oxidized forms of many constituents in water are predominant. Under anoxic conditions in water, reduced forms of chemical species are formed and frequently lead to the release of undesirable odours until oxic conditions develop.
The principal groups of microorganisms in natural water include protists, plants and animals. Some of the physical and biological characteristics of organisms important for water quality considerations are presented in Table 2.5.
Table 2.5 Simplified Classification of Microorganisms in Water (Tchobanoglous and Schroeder, 1985)
|
Kingdom
|
Representative members
|
Size, mm
|
Shape
|
Environmentally resistant stage
|
|
Animal |
Crustaceans
|
|
|
|
|
|
Worms
|
|
|
|
|
|
Rotifers
|
|
|
|
|
Plants
|
Rooted aquatic plants |
|
|
|
|
|
Seed Plants |
|
|
|
|
|
Ferns
|
|
|
|
|
|
Mosses
|
|
|
|
|
Protista
|
Protozoa
|
100-102
|
Variable
|
Cysts
|
|
|
Algae
|
|
|
|
|
|
Fungi (molds and yeasts)
|
100-102
|
Filamentous, coccoid
|
Spores
|
|
|
Blue-green algae
|
100
|
Coccoid, filamentous
|
Cysts
|
|
|
Bacteria
|
10-1-101
|
Rod, coccoid, spiral comma
|
Spores, cystlike
|
Many bacteria, viruses and protozoa are causative organisms for some of the more virulent diseases transmitted to humans directly through water and indirectly through contaminated food.
Assay and confirmation of the presence of the causative agent of waterborne diseases are lengthy and time consuming. Instead of specific analyses, coliform organisms have been used to determine the biological characteristics of natural waters. The coliform group of bacteria are aerobic and/or facultative gram-negative, nonspore-forming, rod-shaped bacteria that ferment lactose to gas. Escherichia coli is commonly used as an indicator organism. This organism is present in the intestine of warm-blooded animals, including humans. Therefore the presence of Escherichia coli in water samples indicates the presence of fecal matter and then the possible presence of pathogenic organisms of human origin. The concentration of indicator organisms is reported in MPN/100 mL (MPN = most probable number) or in CFU/100 mL (CFU = colony forming units).
Other enteric organisms that are also considered indicator organisms are fecal streptococci (Streptococcus faecalis) and clostridia (Clostridium perfringens).
In a typical aquatic ecosystem (Figure 2.1) plant and animal materials are composed of carbon, hydrogen, oxygen, nitrogen, phosphorus and sulfur. These elements are building blocks for carbohydrates, lipids, proteins, phospholipids and nucleic acid.

Figure 2.1 Schematic aquatic ecosystem
Protein and nucleic acids consist of nitrogen, which is required by organisms in greatest quantity after carbon and oxygen. Organic nitrogen ammonia (NH3), nitrite (NO2-), nitrate (NO3-), and nitrogen gas (N2) are important nitrogen-containing compounds in aquatic systems. The atmosphere is the reservoir for nitrogen.
Nitric oxide (NO) is formed during fuel combustion at high temperatures:
N2 + O2 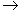 2NO,
2NO, |
(2.16) |
NO reacts easily with ozone and peroxides in the atmosphere:
NO + O3 NO + H2O2 |
(2.17) |
NO2 reacts with OH- in the air to form nitric acid:
NO2 + OH- 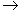 HNO3
HNO3 |
(2.18) |
Nitric acid is a very strong acid, with a large Kw = 101, which results in very fast dissociation as soon as it is in contact with water in the atmosphere:
HNO3 + H2O 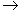 H3O+ + NO3- .
H3O+ + NO3- . |
(2.19) |
All living matters content phosphorus, but its concentration in many natural water environments is low. Phosphoric acid (H3PO4), which is not very volatile, can loose up to 3 H+:
H3PO4 = H+ + H2PO4- ; H2PO4- = H+ + HPO42- ; HPO42- = H+ + PO43-. |
(2.20) |
Sulfate occurs in natural water as organic sulfur, hydrogen sulfide (H2S), elemental sulfur (S) and sulfate (SO42-). Hydrogen sulfide (H2S) is toxic for many organisms and is a source of odour in water. Hydrogen sulfide (H2S) can also combine and precipitate heavy metals such as iron, zinc and cobalt. These metal elements are required for bacterial growth, therefore high levels of H2S may inhibit growth.